Navigation auf uzh.ch
Navigation auf uzh.ch
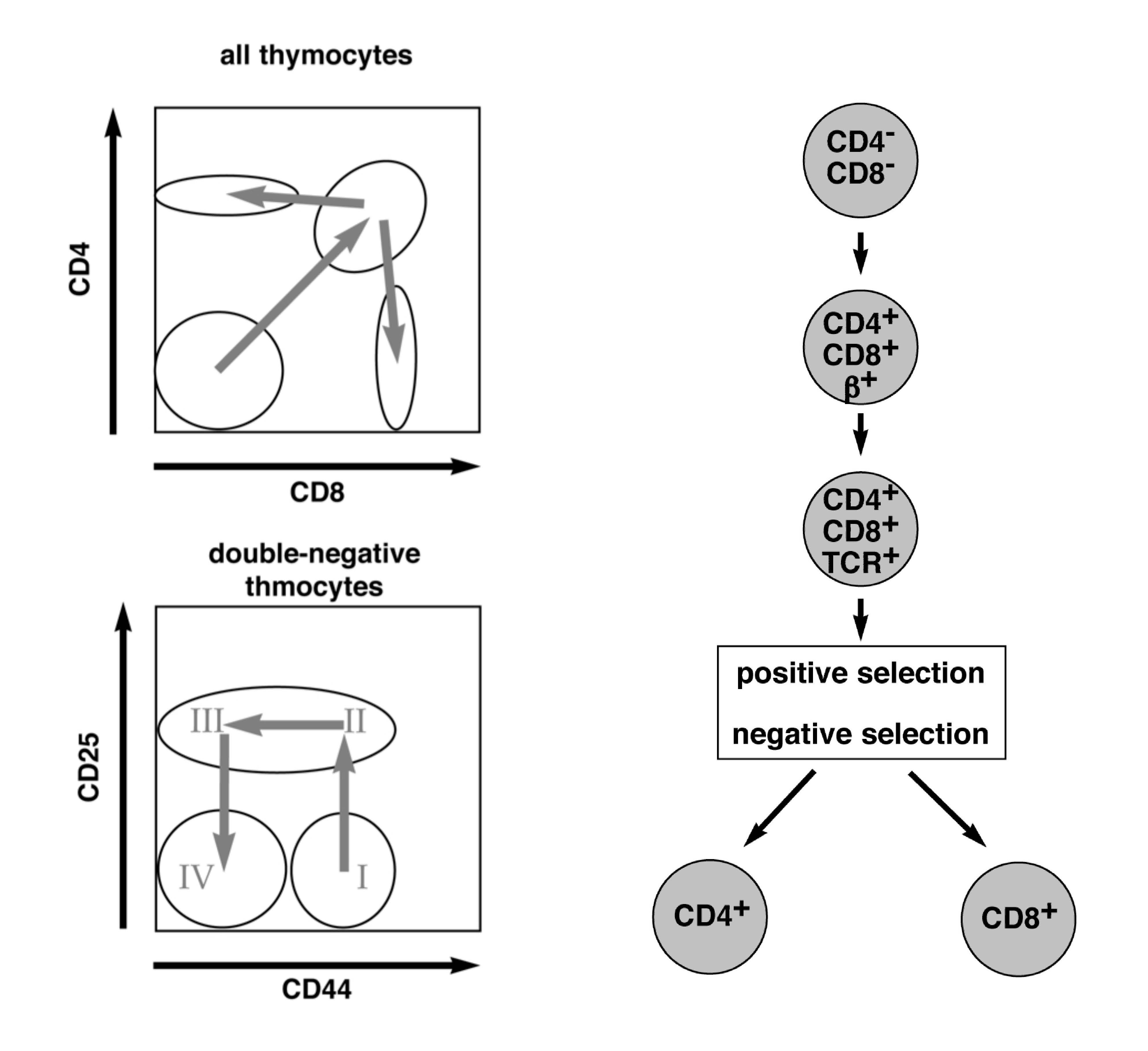
Thymocytes undergo a number of defined developmental steps towards mature T cells. One of them is negative selection, which is important to eliminate self-reactive thymocytes.
Negative selection is controlled by the avidity of the interaction between the T-cell receptor (TCR) and the self-antigen: cells that interact with low avidity to self-antigen can survive while those with high avidity die by apoptosis. Although the exact molecular pathway of negative selection is not yet known, we hypothesize that any transcription factor important for negative selection should be higher expressed in the double positive state of the T cell development than in the remaining states. Following this approach, we have identified one potential gene candidate and are now investigating its importance in T cell development by FACS analysis of different developmental stages, apoptosis and proliferation assays. Therefore, we have generated a transgenic mouse line that specifically overexpresses this gene under the CD4 promoter, and a Cas9/CRISPR mediated knockout strain. We expect to observe massive apoptosis of peripheral T cells within the overexpression model and less negative selection of thymocytes in the knockout mouse model. By following both mouse lines, we aim at gaining additional knowledge of the importance of our gene of interest during T cell development.
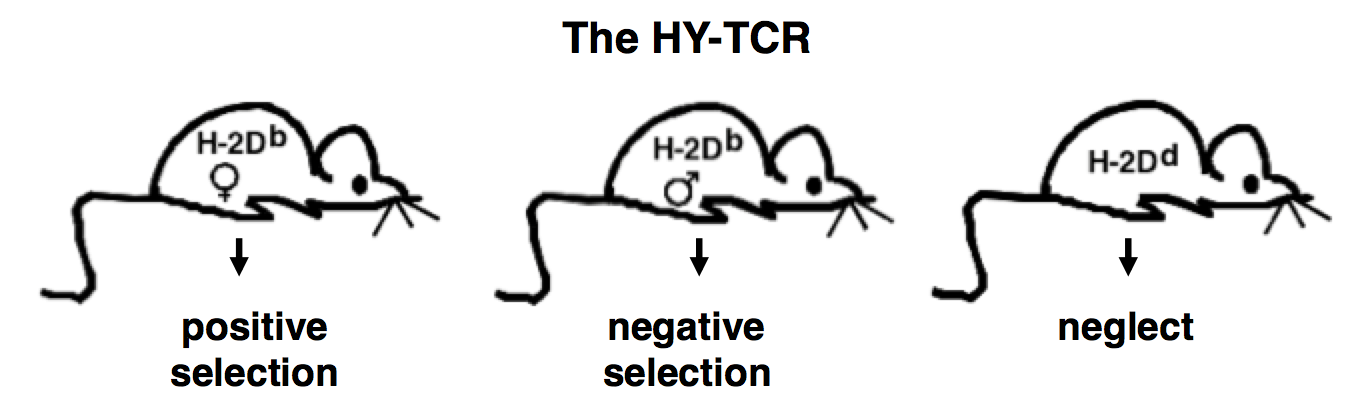
In addition, we investigated whether additional rounds of recombination occur during T cell development upon a negatively selecting signal. To test this hypothesis, we generated by multiple gene targeting a model in which a rearranged TCRα chain is expressed from within the TCRα locus similar to the product of a physiological recombination. In this model we showed that secondary recombination (editing) events cannot rescue developing T cells from negative selection (Buch et al., 2002). Enforcing expression initiation at the CD4+CD8+ stage the hypothesis of editing in T cell development was readdressed in a modification of the model, yielding the same negative result (Kreslavsky et al. 2013). In further experiments we used this insertion transgene to investigate another specific selection checkpoint (β- selection) during earlier thymic development (Croxford et al. 2008). Currently, we address in this model the reasons for premature expression of TCR transgenes. To investigate thymic development further, we generated another mouse strain, in which α-chain expression of the HY-TCR is made inducible. This mouse strain is currently a major research focus in the lab.
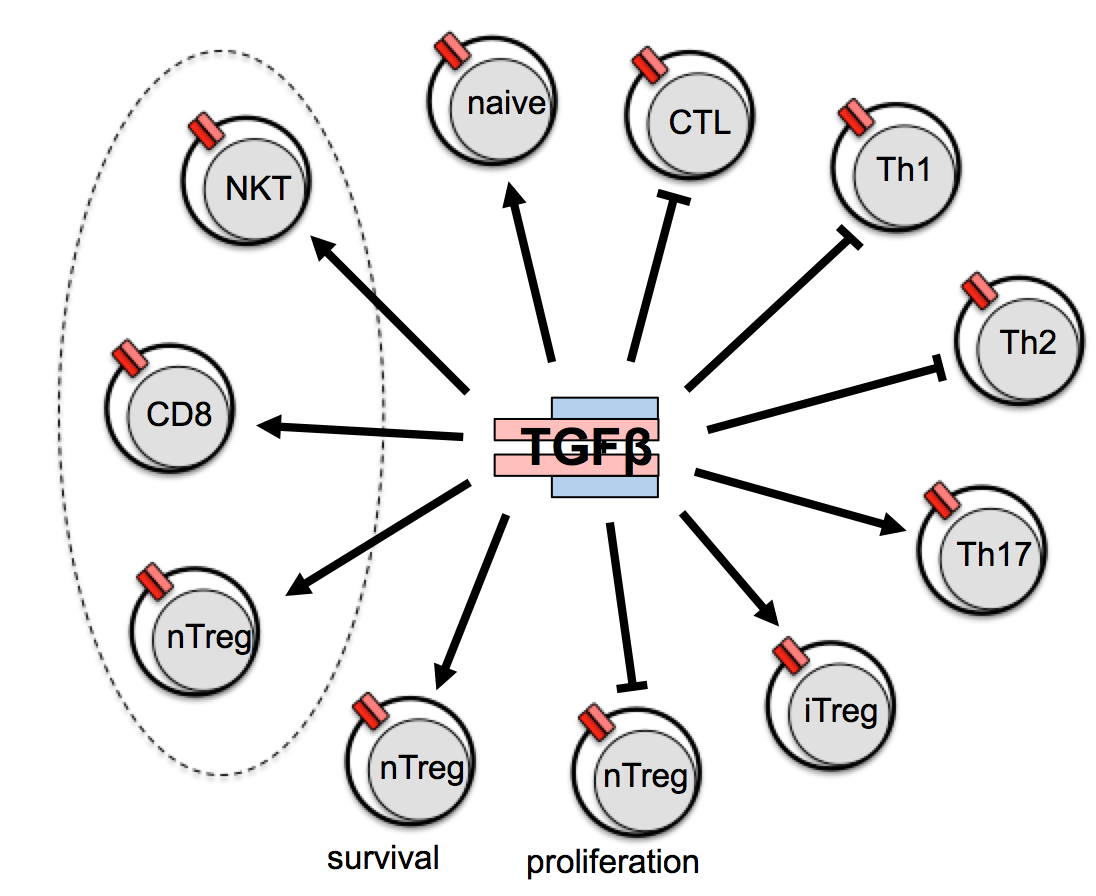
TGF-β1 is a pleiotropically active cytokine and deregulation of TGF-β signaling in T cells results in a deadly systemic autoimmune syndrome. In order to delete TGF-β receptor specifically in mature T lymphocytes we generated by knock-in a CD4-expressing-cell-specific, tamoxifen-inducible Cre-strain (CD4-CreERt2) and crossed it to a mouse strain carrying a loxP- flanked TGFβ receptor gene. In the resulting offspring, we surprisingly failed to observe a decrease in the numbers of regulatory T cells as previously described when TGF-β receptor was ablated by constitutive Cre expression. Also, in contrast to current knowledge, we did not detect a generalized autoimmune syndrome in these mice, thus overturning current thinking about the role of TGF-β in the immune system (Sledzinska et al. 2013). Currently we are working on describing the effects of TGF-β ablation on terminal T cell differentiation.